
Inteded use
QFusion is a system of percutaneous, non-stepped, interspinous stabilization and fusion, designed to promote interspinous arthrodesis, i.e. fusion between the spines of the lumbar spine, in the following cases:
- Degenerative intervertebral disc disease (DDD) (defined as back pain of discogenic origin with disc degeneration, confirmed by clinical history and diagnostic imaging).
- Canal and foraminal stenosis
- Spondylolisthesis
- Traumas
- Tumors

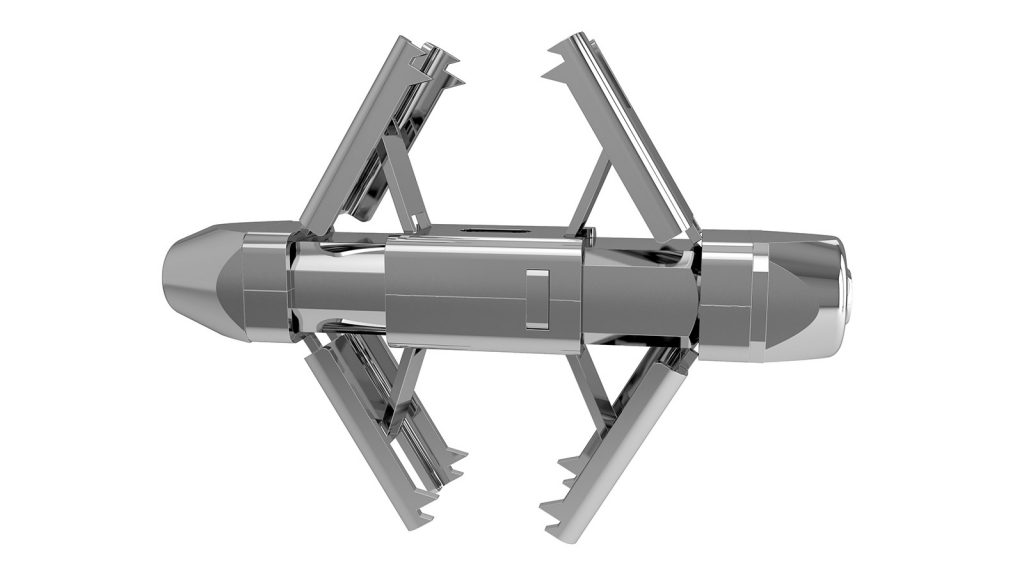
The QFusion interspinous stabilization and fusion system is a percutaneously implantable device that is placed between the spines of the spine.
The device consists of a quadrangular body with four fins placed two by two on the opposite extremities, which, through the compression of the central body, open by raising and approaching simultaneously, until they come into contact with the spines; the anchoring to the spines takes place through the teeth placed at the ends of the fins.
The entire system is made of medical grade titanium; Techlamed guarantees that all devices are manufactured in accordance with ISO 5832-2, ISO 5832-3, ISO 10993-1
Sizes
Available in five variable sizes, the device is made entirely of medical grade titanium.
-
QF08
-
8 mm
-
QF10
-
10 mm
-
QF12
-
12 mm
-
QF14
-
14 mm
-
QF16
-
16 mm
Certification and patents
and it is patented in the European Community and in the most important countries outside Europe.

